Professional Services
Your first step toward global expansion
— we’re with you.
Experience all the services you need.
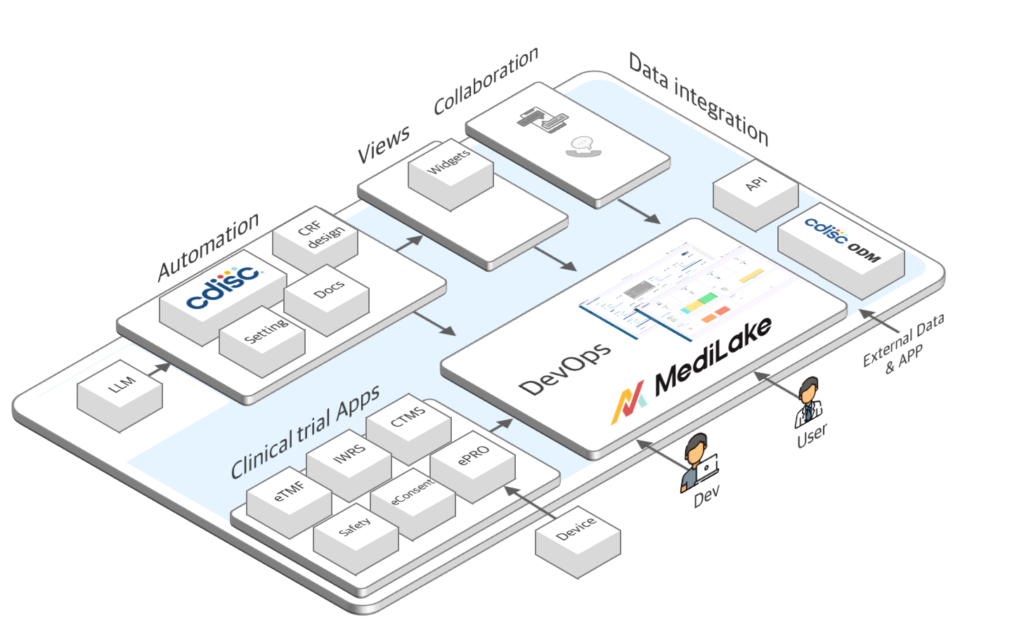
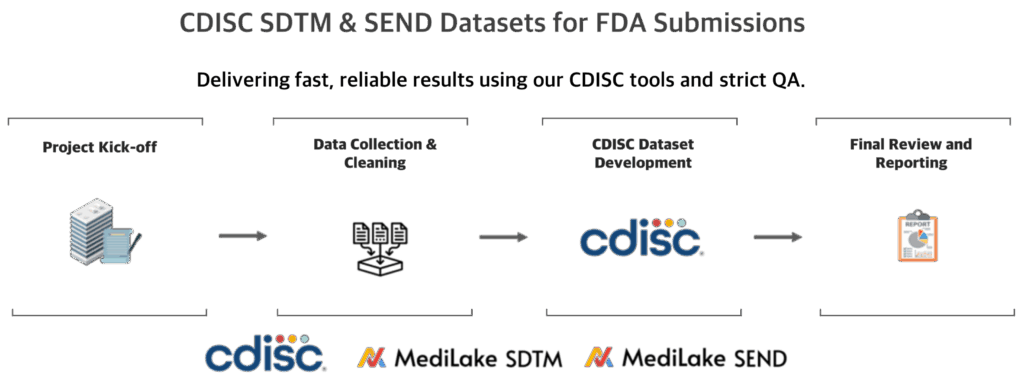
CDISC SEND/SDTM Dataset Creation & End-to-End Clinical Trial Consulting in India
CDISC SEND & SDTM
Data Service
We create CDISC SEND/SDTM datasets,
the international standard for clinical data, enabling compliance
with global regulators and high-quality research.

Service
SDTM/SEND Dataset Services
Data Standardization Consulting
Complete FDA Submission Data Package Services
Building Big Data on CDISC Standards
INDIA Clinical Trial
Consulting
Expand globally, beginning with India.
Expert support from CRO matching to site setup,
ensuring smooth clinical and human application trials..
.

Service
Local CRO Matching in India
Expert Site Selection & Audits in India
On-Site Audits and Study Start-Up Support
CDISC Big Data
Platform Service
Optimized BIG Data Systems for Clinical Trials and Healthcare
We build scalable big data systems based on CDISC standards
,maximizing the value and utility of data across clinical research, healthcare service development operations,and analysis for institutionsresearch centersand analysis for institutions, hospitals, and pharmaceutical companies.
.
.